Colin's Patents
Electrochemical Regeneration of CO₂ Absorbent
To replace heating with fossil fuel in the capture of CO₂, this system uses an electrochemical reactor to recover carbon dioxide and regenerate absorbent hydroxide, with the co-generation of hydrogen. By decomposing water and carbonate, separating reaction products and suppressing their recombination, the reactor offers a sustainable solution for carbon recycling and climate change mitigation.
Mixed-Reactant Fuel Cell
Mixed-reactant fuel cells (MRFCs) are an innovative energy technology that combines fuel and oxygen in a single system to generate electricity. In this study, a solution of potassium formate (fuel) and oxygen (gas) powered a small electric scooter for 15 minutes. The MRFC presents several environmental benefits. With a water soluble fuel it simplifies the design and lowers the cost of fuel cells while producing clean energy and only carbonate as a by-product. Additionally, the fuel, a formate salt or formic acid, can come from renewable sources, further reducing the carbon footprint and offering a sustainable alternative to fossil fuel engines.
Continuous Co-current Electrochemical Reduction of Carbon Dioxide
This invention outlines efficient electrochemical processes for converting carbon dioxide into useful products like formate salts or formic acid. It uses a continuous reactor with a three-dimensional cathode and a two-phase gas/liquid flow, optimizing conditions to achieve high reaction efficiency at low voltages (<10V). Key features include maintaining a high internal gas-to-liquid ratio and operating under mild conditions (pH > 7, CO₂ pressures < 10 bar, and temperatures up to 80°C), making it a promising solution for carbon dioxide reduction and utilization.
Electrochemical Cogeneration of Alkali Metal Halate and Alkaline Peroxide Solutions
The patent presents a process and apparatus for the electrosynthesis of oxygenated halogen salts of alkali metals (such as halates, hypohalites, and perhalates) and an alkaline hydrogen peroxide solution in a single electrochemical reactor. It also introduces a method for oxygen depolarization of cathodes in this process and enables the synthesis of oxygenated chlorine salts without using chromates, enhancing efficiency and sustainability in chemical production.
Perforated Bipole Electrochemical Reactor
A perforated bipole trickle-bed electrochemical reactor is designed for efficient electrosynthesis, especially for producing alkaline hydrogen peroxide—a greener alternative to conventional methods. Its perforated electrodes allow gas (e.g. oxygen) to escape but block parasitic ionic currents between multiple adjacent bipolar cells in the reactor. This eco-friendly design reduces energy use, eliminates hazardous chemicals, and supports sustainable, on-demand peroxide production.
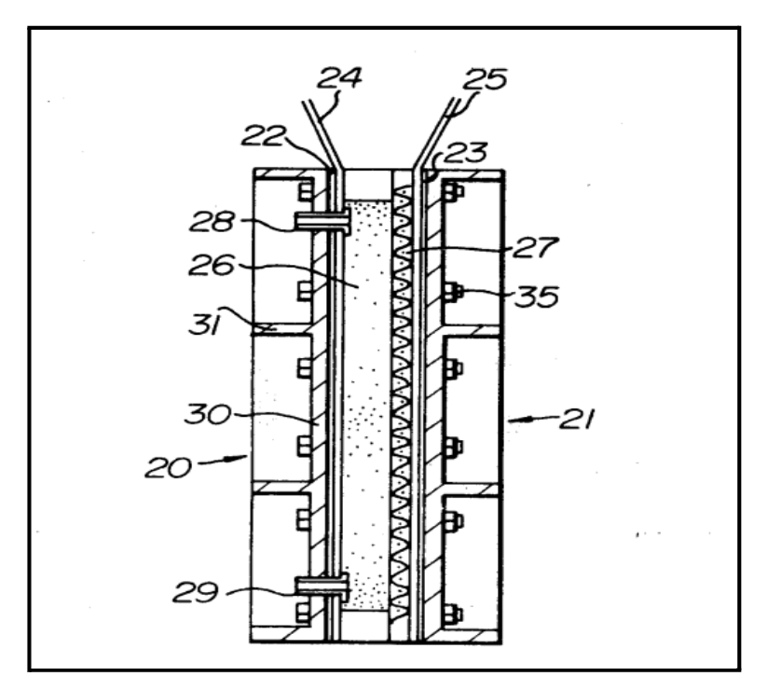
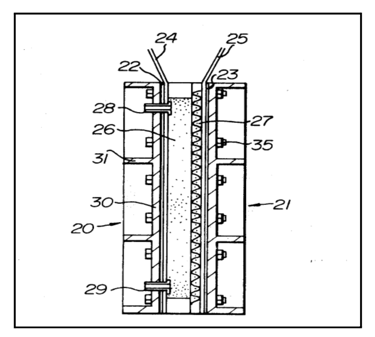
Apparatus for Electrochemical Reactions
The trickle-bed reactor offers a robust alternative to gas-diffusion electrodes for electrochemical processes with sparingly soluble gas reactants. Using two-phase flow through porous electrodes, the trickle-bed achieves a mass transfer capacity greater than 5 per second without concern about electrode flooding.This high mass transfer facility makes trickle-bed reactors suitable for electrochemical operations with gases such as O₂ and CO₂.
Contact Colin Oloman Patents
Inquire about patents related to climate change mitigation and fuel cell technologies by contacting Colin Oloman directly through this section.
colinoloman@gmail.com